Development of new materials that store large quantities of charge and rapidly deliver it on demand is vital to any global transition to a low- or zero-carbon energy economy. My laboratory is taking on the challenge of design principles for fast-charging materials. The fundamental problem is that diffusion of ions (e.g., Li+) through solid materials is slow. Also, materials break down when we force ions through the lattice at fast velocities. If we can develop design principles for robust materials with tailored ionic diffusivity properties, then we can design fast-charging “beyond Li-ion” technologies (e.g., Mg2+ or Al3+) that store more charge than current systems.
How do we get better materials? Exhaustive research into new battery materials has made clear that future innovation will stem from fundamental materials design principles that are “free from the element of art inherent in the fabrication of electrodes. Presently, fabrication is intrinsically heterogeneous, mixing active particles with conductive carbon in a binder matrix. The result is a myriad of distinct interfaces with varying electronic/ionic resistances that complicate data interpretation. What does it mean to say “a material” performs well, when the macroscopic observation might be driven by a tiny fraction of defect sites? We wrestle with questions such as (1) “are the results representative of the entire sample?”; and (2) “how does the composition and structure vary across the sample, and how does that variance influence the properties we measure?”
If we want to understand how changes we make to materials composition and structure influence ionic diffusivity, then what we really need is information at the single-particle scale. Rather than relying on “mix and measure” empiricism, single particle measurements can establish materials design principles by linking known solid state chemistry principles to fundamental ion/electron transport processes that determine charge storage capacity and rate.2 The problem is that single particle measurements are often slow and tedious. To overcome the low-throughput obstacle, my lab developed a widefield light microscopy approach that images hundreds of particles in a single experiment.
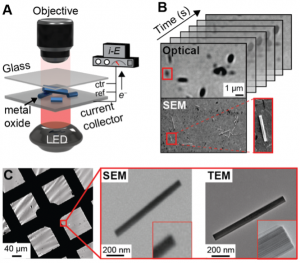
Our fundamental insight into this challenge was the recognition that relevant battery materials (graphite, LiCoO2, etc.) exhibit subtle color changes during cycling that can be direct and real-time reporters of redox changes in the material. Our innovative light microscopy method (panel A above) links these detectable color changes to underlying elemental changes in the solid (e.g, W6+/W5+ redox in WO3). Importantly, these single nanoparticle measurements avoid the need for additives and binders that typically complicate data interpretation. We extract Li-ion diffusivity from the time-dependent data and correlate it with particle structure using AFM, SEM, and TEM (panels B-C). We have used this approach to reveal (1) structure/property relationships of WO3 nanoparticles in electrochromic smart windows: long nanorods turn darker due to exposed planes with open square window Li-ion binding sites; (2) Li-ion trapping at particle-particle interfaces diminishes cycle performance; and (3) the charge storage mechanism in single WO3 nanoparticles. Based on our signal-to-noise ratio analysis, we can observe Li-ion insertion dynamics in 13 nm-diameter WO3 particles even with a conventional light microscope. Such straightforward and high-throughput measurements can be used to diagnose the state of charge and failure mechanisms of electrodes. The widefield approach exceeds the throughput of serial measurements that attach battery particles to sharp tip electrodes. In summary, my lab has developed a new optical technique to study Li-ion insertion dynamics in single nanoparticles and is well-positioned to make breakthrough discoveries in electrochemical energy storage.

